Shandong Xinbo assists Baike Biotechnology in obtaining clinical trial approval for its Class 1 new drug, CBB1 injection, a natural all human monoclonal antibody against rabies virus
2023/03/15
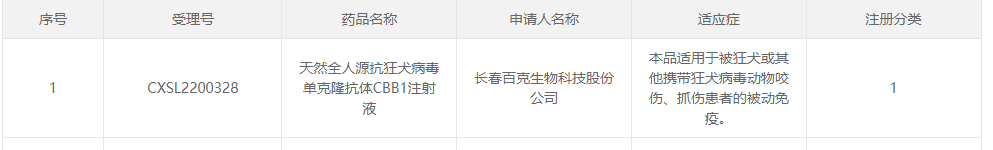
Recently, Changchun Baike Biotechnology Co., Ltd.'s Class 1 new drug - natural all human monoclonal antibody CBB1 injection against rabies virus - has been approved by the National Medical Products Administration (NMPA) of China.
Shandong Xinbo Pharmaceutical Research Co., Ltd. is committed to the preclinical safety evaluation of biopharmaceuticals, traditional Chinese medicine, chemical drugs, and medical devices, providing standardized and comprehensive preclinical solutions for innovative pharmaceutical enterprises and assisting in the drug development process.
The Drug Safety Evaluation Research Center of Shandong Xinbo Pharmaceutical R&D Co., Ltd. has undertaken the tissue crossover study, in vitro cytokine release study, methodological establishment and validation, tissue distribution study, and preclinical safety evaluation of a Class 1 new drug - natural all human anti rabies virus monoclonal antibody CBB1 injection. This has helped Baike Biotech obtain clinical trial approval for its natural all human anti rabies virus monoclonal antibody CBB1 injection project. If the antibody variety successfully completes clinical trials and is approved for market, it will become the second monoclonal antibody drug for the treatment of rabies virus in China, following the Omotevir monoclonal antibody injection.
The Drug Safety Evaluation Research Center of Shandong Xinbo Pharmaceutical R&D Co., Ltd. has undertaken the tissue crossover study, in vitro cytokine release study, methodological establishment and validation, tissue distribution study, and preclinical safety evaluation of a Class 1 new drug - natural all human anti rabies virus monoclonal antibody CBB1 injection. This has helped Baike Biotech obtain clinical trial approval for its natural all human anti rabies virus monoclonal antibody CBB1 injection project. If the antibody variety successfully completes clinical trials and is approved for market, it will become the second monoclonal antibody drug for the treatment of rabies virus in China, following the Omotevir monoclonal antibody injection.