General toxicological studies
 Service items
Service items
■ Single dose toxicity test (rodents and non rodents)
■ Repeated dose toxicity test (rodents and non rodents)
Animal species: including dogs, pigs, non-human primates
Route of administration:
·Oral (gavage, capsule, food), nasal drip
·Intramuscular injection, subcutaneous injection, intraperitoneal injection, intradermal injection
·Intravenous injection (injection and infusion)
·Eye medication, inhalation medication
·Other routes of medication: electroporation, intra-articular injection, thalamic injection, percutaneous administration, etc
■ Repeated dose toxicity test (rodents and non rodents)
Animal species: including dogs, pigs, non-human primates
Route of administration:
·Oral (gavage, capsule, food), nasal drip
·Intramuscular injection, subcutaneous injection, intraperitoneal injection, intradermal injection
·Intravenous injection (injection and infusion)
·Eye medication, inhalation medication
·Other routes of medication: electroporation, intra-articular injection, thalamic injection, percutaneous administration, etc
 Service advantages
Service advantages
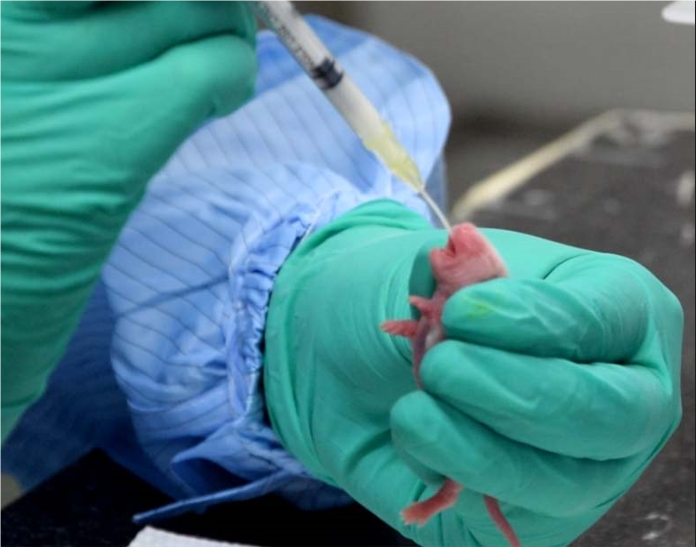
>Pediatric drug safety evaluation
The center has experience in safety evaluation technology of experimental systems such as young rats (3 days old) and dogs (7 days old), and has completed evaluation of several pediatric drugs.
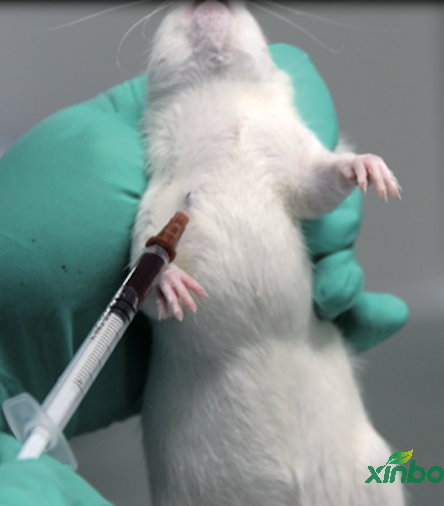
>Clinical evaluation of long-term intravenous drip medication
Long time intravenous drip administration technology for conscious rats and dogs can be used for continuous intravenous administration for 24 hours. The center has established the technique of continuous intravenous drip for 3 months in conscious rats and dogs.
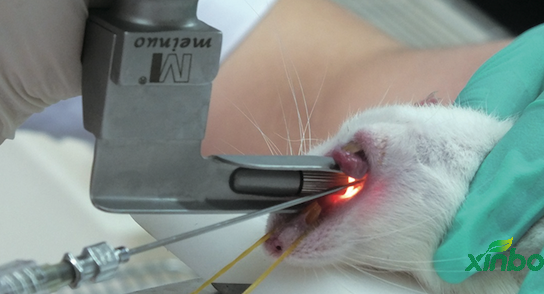
>Safety evaluation of inhalation medication
The drug was administered by oral tracheal intubation in conscious rats and dogs. The administration process was rapid and accurate, and the animal injury was small.
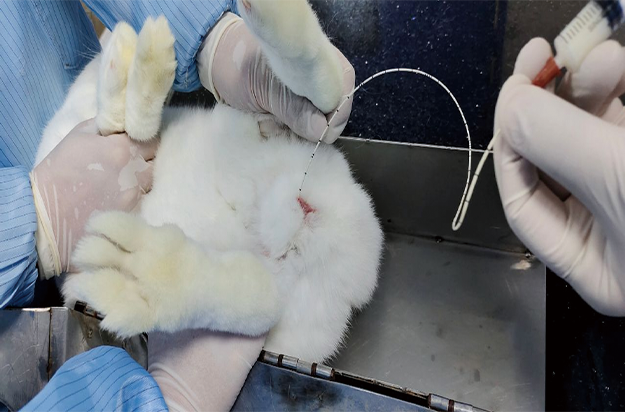
>Jugular sinus blood collection technology
The animals do not need anesthesia, with small trauma, in line with animal ethics, and can meet the requirements of PK/TK blood collection.
>Monkey neurotoxicity test:
The successful rate of injection was 100%, and the nerve toxicity test was completed.
The center has experience in safety evaluation technology of experimental systems such as young rats (3 days old) and dogs (7 days old), and has completed evaluation of several pediatric drugs.
>Clinical evaluation of long-term intravenous drip medication
Long time intravenous drip administration technology for conscious rats and dogs can be used for continuous intravenous administration for 24 hours. The center has established the technique of continuous intravenous drip for 3 months in conscious rats and dogs.
>Safety evaluation of inhalation medication
The drug was administered by oral tracheal intubation in conscious rats and dogs. The administration process was rapid and accurate, and the animal injury was small.
>Jugular sinus blood collection technology
The animals do not need anesthesia, with small trauma, in line with animal ethics, and can meet the requirements of PK/TK blood collection.
>Monkey neurotoxicity test:
The successful rate of injection was 100%, and the nerve toxicity test was completed.