Biological evaluation of medical devices
 Service items
Service items
■ In vitro cytotoxicity test
■ Systemic toxicity test
■ Post implantation local reaction test
■ Stimulation and delayed type hypersensitivity test
■ Genotoxicity test
■ Reproductive toxicity test
■ Pre clinical animal safety and effectiveness test of medical devices
■ Non clinical animal test of medicine and machinery combination project
■ Systemic toxicity test
■ Post implantation local reaction test
■ Stimulation and delayed type hypersensitivity test
■ Genotoxicity test
■ Reproductive toxicity test
■ Pre clinical animal safety and effectiveness test of medical devices
■ Non clinical animal test of medicine and machinery combination project
 Service advantages
Service advantages
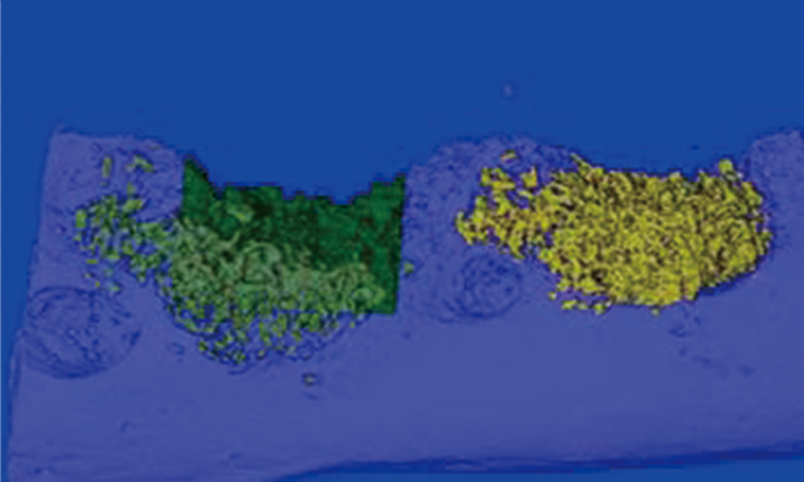
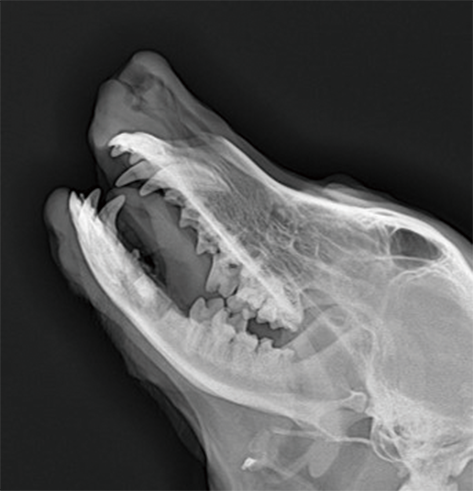
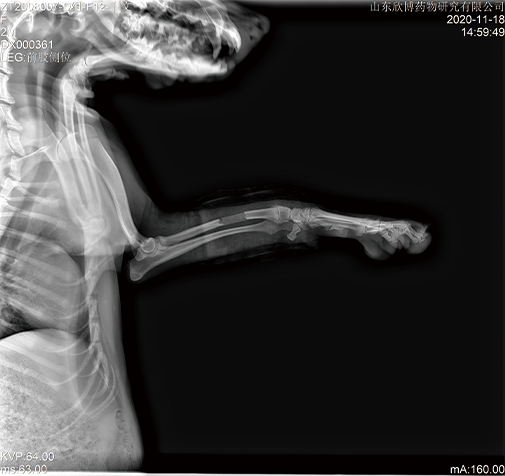
Non clinical animal tests of high-end medical devices have been completed in GLP laboratory according to regulatory requirements. Animal test studies on the safety and effectiveness of several three types of medical devices have been completed, including in vivo hemostatic materials, neurosurgical hemostatic materials, intravascular blocking or hemostatic materials, dental implant materials, orthopedic implant materials, new skin suture materials, etc., and have passed the review.