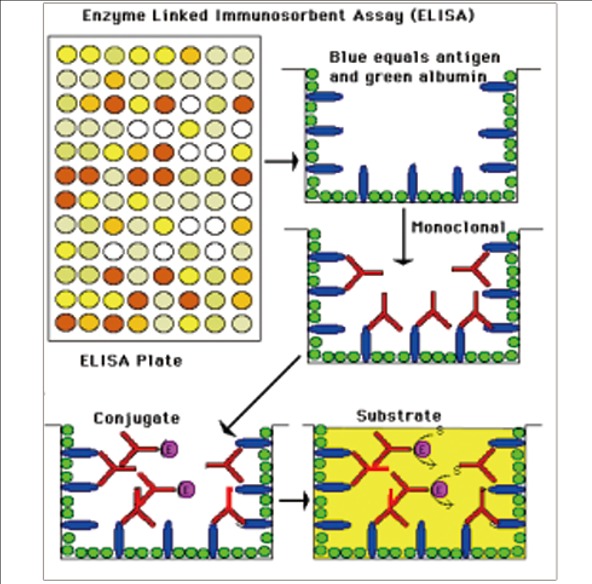
Immunogenicity Study
 Service items
Service items
■ Immunogenicity (ADA) Method Development: Indirect ELISA, Bridging ELISA, ACEELISA, etc.
■ Antinuclear Antibody Detection Test
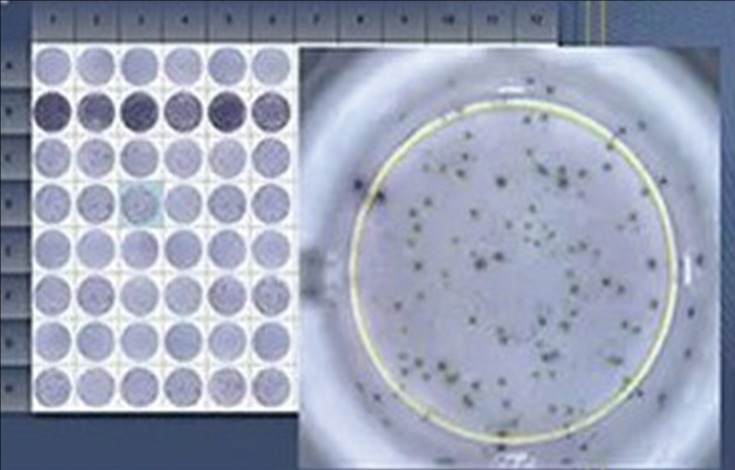
■ Enzyme-Linked Immunospot Assay (ELISPOT)
■ Soft Agar Colony Formation Assay
■ Antinuclear Antibody Detection Test
■ Enzyme-Linked Immunospot Assay (ELISPOT)
■ Soft Agar Colony Formation Assay
 Service advantages
Service advantages
>Based on the characteristics of the drug, personalized detection schemes are designed, including but not limited to direct ELISA, indirect ELISA, bridging ELISA, and ACE ELISA methods. Method development and validation are conducted according to GLP standards and the "Guideline on Immunogenicity Assessment of Biotechnology-Derived Therapeutic Proteins." A multi-tiered detection strategy is employed for sample testing, sequentially performing screening assays, confirmatory assays, and, if necessary, titer assays and/or neutralization assays for confirmed positive samples.
>The laboratory has successfully conducted immunogenicity testing for dozens of drugs, establishing detection methods for various macromolecular drugs such as peptides, short peptides, monoclonal antibodies, and conjugated antibodies. For monoclonal antibody drugs, under specific test conditions, drug resistance can reach up to 600 µg/ml or higher.
>The lab has a comprehensive in vitro cytokine release detection platform. Using human whole blood or human peripheral blood mononuclear cells, the platform evaluates cell activation and cytokine release in vitro, which can, to some extent, compensate for the inability of animal models to fully simulate the human immune response due to species differences.
>The laboratory has successfully conducted immunogenicity testing for dozens of drugs, establishing detection methods for various macromolecular drugs such as peptides, short peptides, monoclonal antibodies, and conjugated antibodies. For monoclonal antibody drugs, under specific test conditions, drug resistance can reach up to 600 µg/ml or higher.
>The lab has a comprehensive in vitro cytokine release detection platform. Using human whole blood or human peripheral blood mononuclear cells, the platform evaluates cell activation and cytokine release in vitro, which can, to some extent, compensate for the inability of animal models to fully simulate the human immune response due to species differences.