Medical device safety evaluation platform
 Service items
Service items
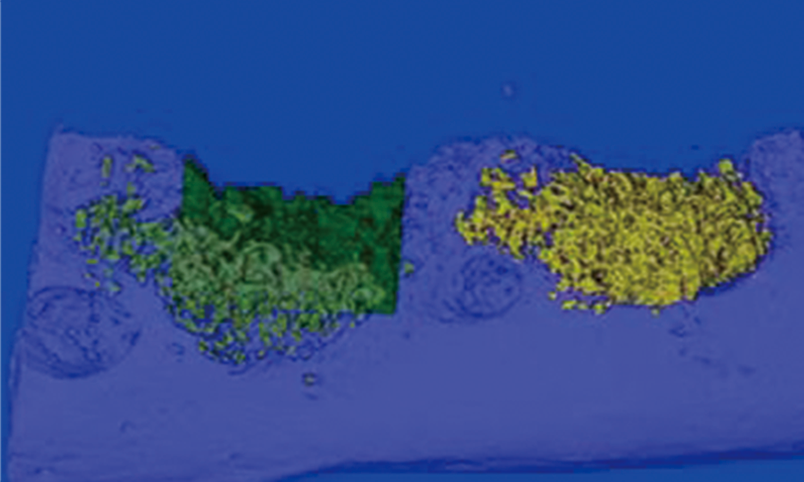
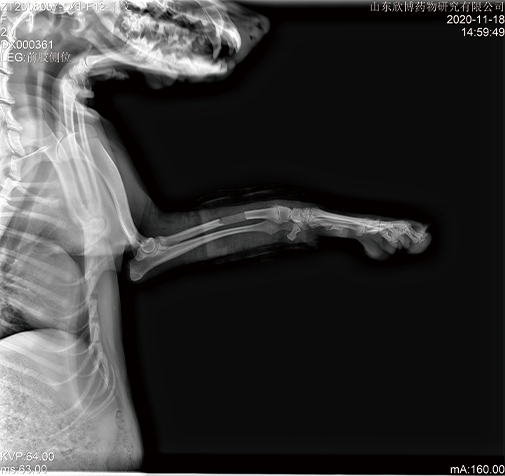
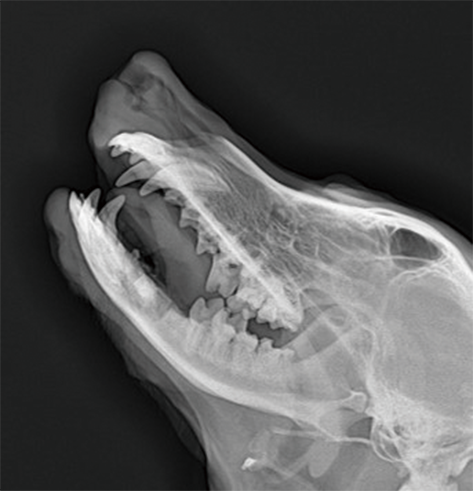
Non clinical animal tests of high-end medical devices have been completed in GLP laboratory according to regulatory requirements. Animal test studies on the safety and effectiveness of several three types of medical devices have been completed, including in vivo hemostatic materials, neurosurgical hemostatic materials, intravascular blocking or hemostatic materials, dental implant materials, orthopedic implant materials, new skin suture materials, etc., and have passed the review.
■ In vitro cytotoxicity test
■ Systemic toxicity test
■ Post implantation local reaction test
■ Stimulation and delayed type hypersensitivity test
■ Genotoxicity test
■ Reproductive toxicity test
■ Pre clinical animal safety and effectiveness test of medical devices
■ Non clinical animal test of medicine and machinery combination project
■ In vitro cytotoxicity test
■ Systemic toxicity test
■ Post implantation local reaction test
■ Stimulation and delayed type hypersensitivity test
■ Genotoxicity test
■ Reproductive toxicity test
■ Pre clinical animal safety and effectiveness test of medical devices
■ Non clinical animal test of medicine and machinery combination project
 Evaluated varieties
Evaluated varieties
Brain implantation: absorbable hemostatic gauze, absorbable dural sealing medical glue, Fuaile medical glue
>Organ implantation: Kangpaite medical glue, Mefalen sustained-release implant
Dental materials: injectable root filling paste, root canal filling materials
Nasal implantation: biodegradable ear nose styptic sponge
>Muscle pocket implantation: active biological bone
Skin implantation: biological amnion/chorion
>Spine implantation: absorbable hemostatic fluid gelatin
>Subcutaneous implantation: dural medical glue
>Organ implantation: Kangpaite medical glue, Mefalen sustained-release implant
Dental materials: injectable root filling paste, root canal filling materials
Nasal implantation: biodegradable ear nose styptic sponge
>Muscle pocket implantation: active biological bone
Skin implantation: biological amnion/chorion
>Spine implantation: absorbable hemostatic fluid gelatin
>Subcutaneous implantation: dural medical glue
 Company performance - obtaining clinical approval(Only some Company performance are displayed)
Company performance - obtaining clinical approval(Only some Company performance are displayed)
| Drug name | Acceptance No | Indication | Class |
| Use a high -frequency cut operation at one time | National Inspection 20233011227 | This product is used with softening gastrointestinal endoscopy and high -frequency surgical equipment, and uses high -frequency current to cut the tissue in the digestive tract. | Third category |
| Active bone bone | National Inspection 20223131334 | It is used to fill bone defects caused by trauma or surgery and does not affect the stability of the bone structure. | Third category |
| Medical glue | National Inspection 20213020696 | It is used for the closure of surgical incision close to the edge of the skin, including the closure of the minimally invasive intervention surgery puncture. After laparoscopic debridement, spray woun | Third category |
| Absorbing blood vessel seal medical glue | National Inspection 20193020081 | When it is used for blood vessel reconstruction, hemostasis can be assisted through mechanical closure. | Third category |
| Absorbing hardener seal medical glue | National Inspection 20183020031 | It is used for auxiliary seal at the sutures of the hard meningiomy during craniotomy to prevent leakage of the cerebrospinal fluid. During the reconstruction of vascular reconstruction, hemostasis is | Third category |
| Tissue glue | National Inspection 20173024576 | For the treatment of stomach vein rosor embolism. | Third category |
| Tissue adhesion | National Inspection 20173023182 | This product is only used for the use of the body table for local application, which is limited to the combined surgical incision on the edge pair, including the minimally invasive intervention surger | Third category |
| Medical glue | National Inspection 20153021282 | It is used for the closure of surgical incision close to the edge of the skin, including the closure of the minimally invasive intervention surgery puncture. After a laparoscopic debridement, spray wo | Third category |