Safety evaluation platform for cell therapy products
 Service items
Service items
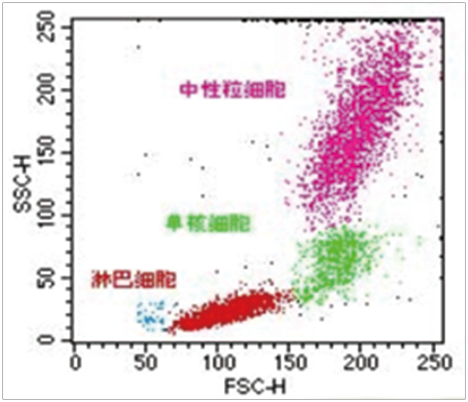
The safety evaluation of cell therapy can be carried out. At present, pharmacodynamic and pharmacokinetic studies of NKT cells, MSC cells and CAR-T cells and a full set of pre clinical safety evaluation have been carried out.
■ Cell count
■ Pharmacodynamic study
■ Single dose toxicity test (rodents)
■ Repeated dose toxicity test (rodents)
■ Safety pharmacology test
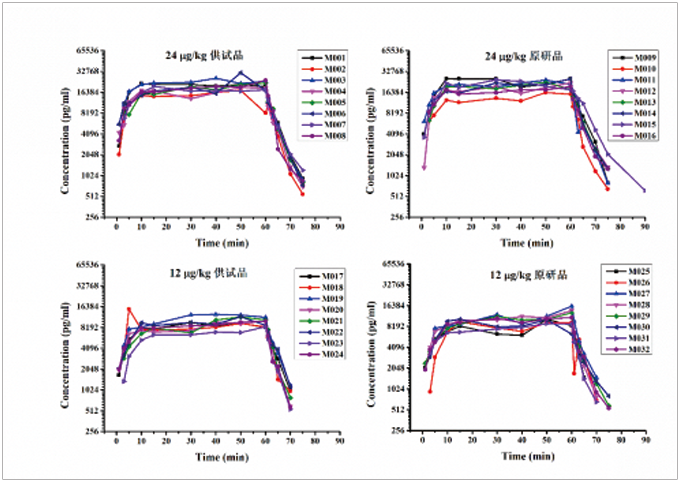
■ Pharmacokinetic test (including methodological establishment and validation)
■ Local toxicity test
■ Immunogenicity test (including methodological establishment and validation)
■ Tumorigenicity test
■ Tumor forming test
■ Soft agar cloning test
■ Cell count
■ Pharmacodynamic study
■ Single dose toxicity test (rodents)
■ Repeated dose toxicity test (rodents)
■ Safety pharmacology test
■ Pharmacokinetic test (including methodological establishment and validation)
■ Local toxicity test
■ Immunogenicity test (including methodological establishment and validation)
■ Tumorigenicity test
■ Tumor forming test
■ Soft agar cloning test
 Company performance - obtaining clinical approval(Only some Company performance are displayed)
Company performance - obtaining clinical approval(Only some Company performance are displayed)
| Drug name | Acceptance No | Indication | Class |
| GKL-006 injection solution | CXSL2300567 | Used to treat irregular primary liver cell carcinoma | 1 |