Genetic Toxicity Study
 Service items
Service items
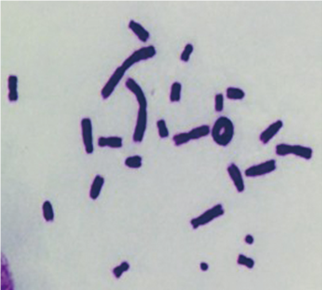
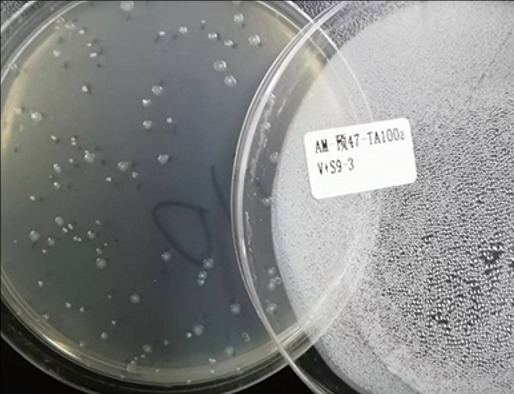
■ Bacterial Reverse Mutation (Ames) Test
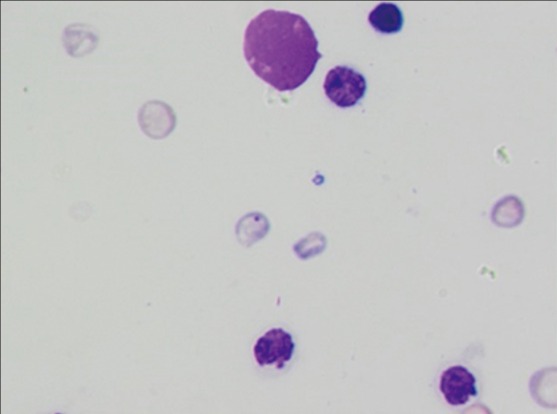
■ In Vitro Mammalian Cell Chromosome Aberration Test
■ In Vitro Mouse Lymphoma Cell Tk Gene Mutation Assay
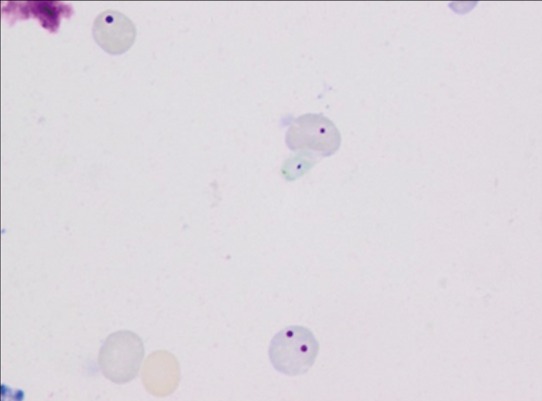
■ In Vivo Mammalian Micronucleus Test
■ Mouse Spermatogonial Chromosome Aberration Test
■ In Vitro Micronucleus Test
■ Mini-Ames Test
■ Cytotoxicity Screening Assay
■ In Vitro Mammalian Cell Chromosome Aberration Test
■ In Vitro Mouse Lymphoma Cell Tk Gene Mutation Assay
■ In Vivo Mammalian Micronucleus Test
■ Mouse Spermatogonial Chromosome Aberration Test
■ In Vitro Micronucleus Test
■ Mini-Ames Test
■ Cytotoxicity Screening Assay
 Service advantages
Service advantages
>The study director has over 10 years of experience conducting genetic toxicity studies.
> The genetics team has experience in designing studies for conventional drugs, drug impurities, and peptide drugs.
>The genetics laboratory is equipped with advanced instruments such as fluorescence microscopes, inverted microscopes, CO₂ incubators, and biosafety cabinets.
>The facility includes GLP-compliant cell culture and microbiology rooms to prevent cross-contamination.
> The genetics team has experience in designing studies for conventional drugs, drug impurities, and peptide drugs.
>The genetics laboratory is equipped with advanced instruments such as fluorescence microscopes, inverted microscopes, CO₂ incubators, and biosafety cabinets.
>The facility includes GLP-compliant cell culture and microbiology rooms to prevent cross-contamination.