Shandong Xinbo Helps China's First Three Target DNA Tumor Vaccine - NMM Tumor Therapeutic DNA Vaccine Naked Plasmid Injection Obtain Clinical Trial License
2023/04/07
Recently, the Class 1 new drug - NMM tumor therapeutic DNA vaccine naked plasmid injection (acceptance number CXSL2250571) developed by Gu'an Dingtai Haigui Biotechnology Co., Ltd. has been approved by the National Medical Products Administration (NMPA) of China, with indications for advanced solid tumors and relapsed, refractory lymphoma.
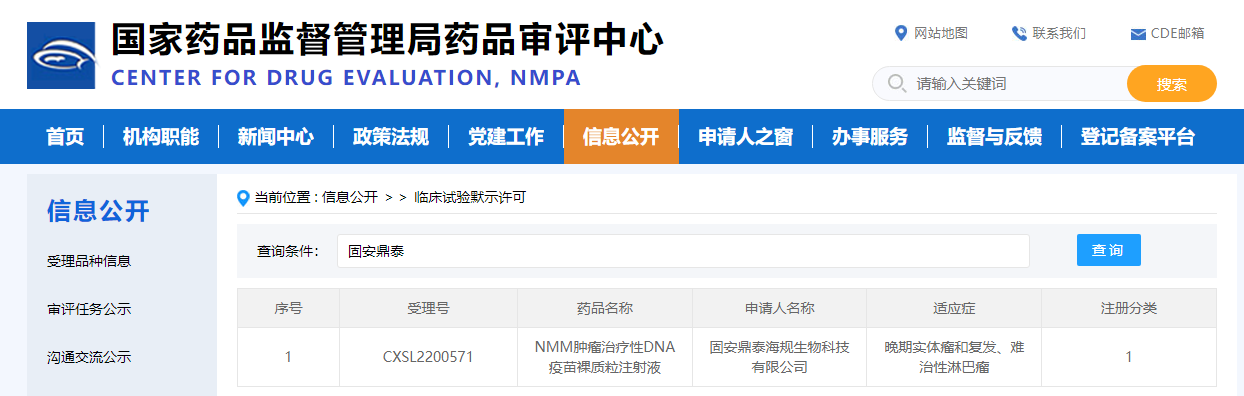
NMM tumor therapeutic DNA vaccine is a novel immunotherapy drug that can generate highly efficient systemic anti-tumor immune response through local injection. It has good safety and tolerability, and has the potential to develop into an effective anti-tumor recurrence drug. It is the first three target DNA tumor vaccine in China.
Shandong Xinbo Pharmaceutical Research Co., Ltd. is committed to the preclinical safety evaluation of biopharmaceuticals, traditional Chinese medicine, chemical drugs, and medical devices, providing standardized and comprehensive preclinical solutions for innovative pharmaceutical enterprises and assisting in the drug development process.
The Drug Safety Evaluation Research Center of Shandong Xinbo Pharmaceutical Research Co., Ltd. has established a long-term cooperative relationship with Gu'an Dingtai Haigui Biotechnology Co., Ltd. It has undertaken the methodological establishment and validation, tissue distribution research, and complete preclinical safety evaluation of the first class new drug - NMM tumor therapeutic DNA vaccine naked plasmid injection, helping the Gu'an Dingtai Haigui NMM tumor therapeutic DNA vaccine naked plasmid injection project to obtain clinical trial approval. It is the first three target DNA tumor vaccine in China.
