Shandong Xinbo was invited to participate in the first National Experimental Pathology High end Medical Device Innovation Forum
2023/05/17
Shandong Xinbo Pharmaceutical R&D Co., Ltd. has been invited to participate in this grand event. On June 10th from 14:00 to 14:20, Bu Wen, the head of Shandong Xinbo Medical Device Evaluation Department, will give a speech on the theme of "Ethical Requirements for Animal Testing of Medical Devices". We sincerely invite colleagues in the industry to attend this conference and communicate with guests. At the same time, we have set up booth B21 and welcome everyone to visit the booth to obtain the latest materials and draw exquisite gifts.

Shandong Xinbo Pharmaceutical Research Co., Ltd. was established in April 2010. It is a national high-tech enterprise, Shandong Province Enterprise Technology Center, Shandong Province Gazelle Enterprise, and the only scientific research service-oriented enterprise in the province that integrates pharmacological research, toxicology research, drug analysis and testing services, animal pathology testing, and medical device evaluation services.
Since its establishment, Shandong Xinbo has been actively conducting biological evaluations of medical devices and has completed nearly a hundred evaluation projects in accordance with GLP requirements, such as absorbable dura mater sealing medical adhesive, absorbable vascular sealing medical adhesive, alpha cyanoacrylate medical adhesive, injectable root filling paste, disposable absorbable nail intradermal stapler, needle free injector, active biological bone repair materials (drug containing devices), calcium silicon bioceramic bone, absorbable hemostatic fluid gelatin, degradable ear and nose hemostatic sponge, biological amniotic membrane chorion, absorbable hemostatic gauze, dura mater sealing adhesive, disposable high-frequency cutting knife, etc. In addition to conducting experiments on the biological evaluation of medical devices according to GB/T 16886, Multiple animal experiments on medical devices were conducted based on the characteristics of each device (excluding biocompatibility experiments not covered in the GB/T16886 series standards) to evaluate the safety, effectiveness, and feasibility of medical devices.

Shandong Xinbo invested 50 million yuan to establish a medical device safety evaluation research center, which will be officially put into use in 2022. The Medical Device Safety Evaluation Research Center has a total construction area of 6000 square meters, mainly including a large animal laboratory, animal operating room, functional laboratory, barrier animal laboratory, small animal laboratory, and dissection room. It purchases CT, pet DR, veterinary ultrasound diagnostic instrument, animal respiratory anesthesia machine, monitor, high-frequency electric knife, oxygen generator, rat brain locator, electric sterilization pot, card type steam sterilizer, ozone disinfection cabinet, binocular indirect ophthalmoscope, slit lamp microscope, biosafety cabinet, biochemical incubator, carbon dioxide incubator, flow cytometer, PCR gene amplifier, fluorescence quantitative PCR instrument, ultra micro ultraviolet spectrophotometer, fully automatic blood biochemistry analyzer, fully automatic blood analyzer, coagulation analyzer, high-performance liquid chromatography, mass spectrometer. There are a total of 748 sets of instruments waiting.
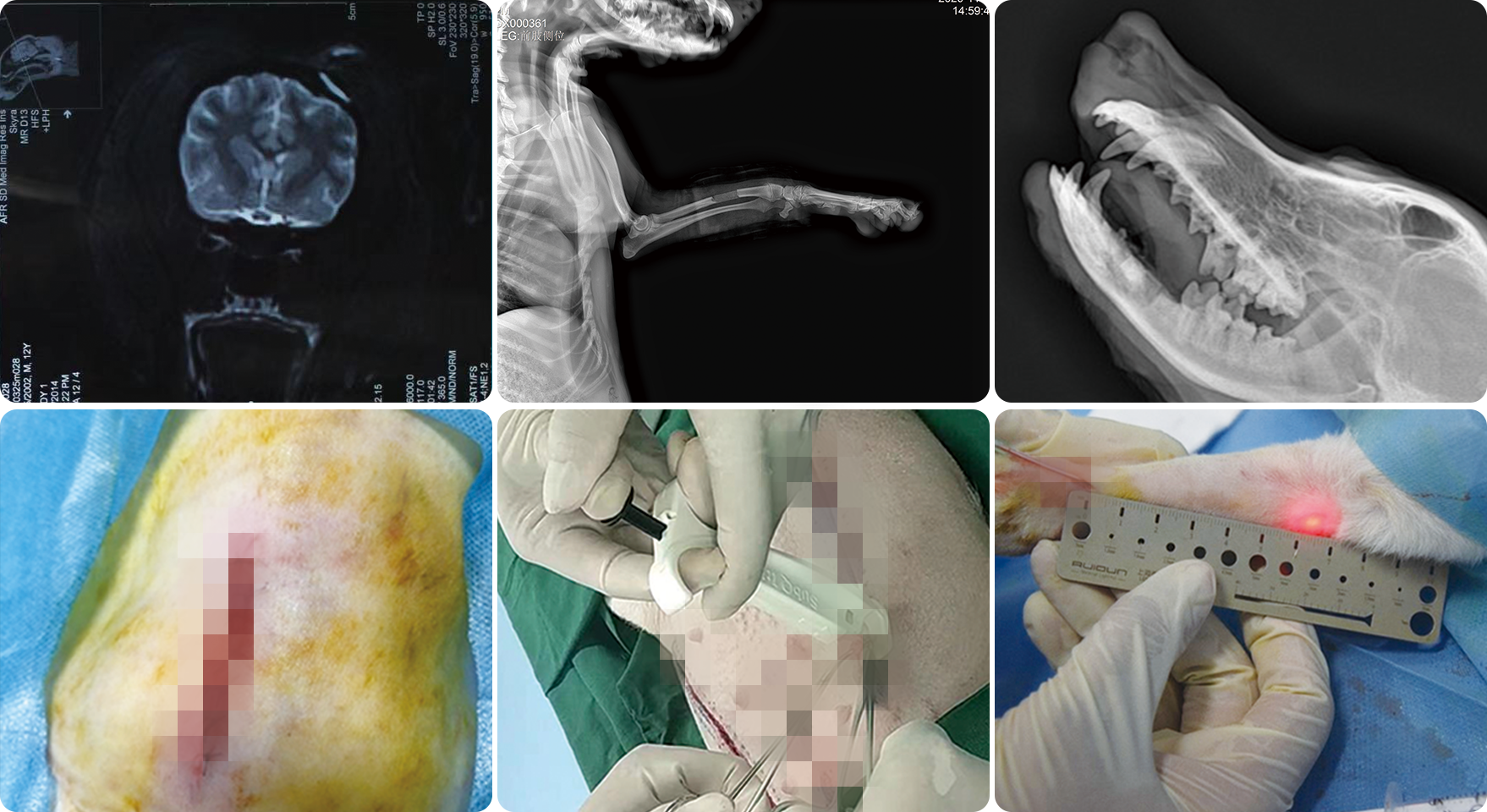
The large animal operating room strictly controls aseptic operations, provides independent air supply, and can perform two surgeries simultaneously. There are 32 large animal laboratories that can accommodate over 1000 dogs, 400 pigs, and 400 monkeys simultaneously. There are 66 small animal laboratories that can accommodate over 7500 mice, 500 rabbits, and 4000 guinea pigs simultaneously.

