Shandong Xinbo supports the approval of the world's first PLpro small molecule inhibitor for clinical use
2023/09/22
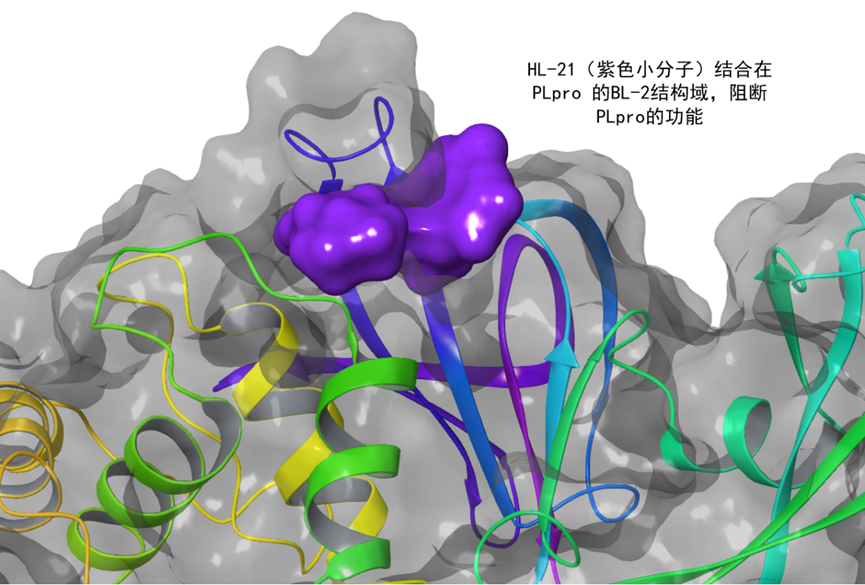
On August 29, 2023, the Papain Like Protease (PLpro) small molecule inhibitor HL-21, jointly developed by He Wei and his team from the School of Pharmacy at Tsinghua University and the Land team from the School of Basic Medicine at Fudan University, was approved and issued the "Drug Clinical Trial Approval Notice" by the National Medical Products Administration, becoming the world's first PLpro inhibitor drug to enter clinical development.
The Drug Safety Evaluation Research Center of Shandong Xinbo Pharmaceutical Research Co., Ltd. (hereinafter referred to as Shandong Xinbo) undertook the preclinical safety research of HL-21 and helped it obtain clinical trial approval.
The Tsinghua University team adopted fragment based drug discovery and structure based drug design strategies, and utilized a novel PLpro active probe technology to discover a class of structurally novel, highly active, and selective PLpro small molecule inhibitors. HL-21 exhibits high affinity, high enzyme activity inhibition, and high inhibitory activity against various coronavirus strains (such as SARS-COV-2, HCoV-OC43, HCOV-229E, HCOV-NL63, etc.), as well as pharmacological effects in animal infection models. It also demonstrates many advantages in terms of dosage, safety, cost, and potential application scenarios.
As an anti coronavirus drug target different from Mpro (main hydrolase) and RdRp (RNA polymerase), PLpro not only plays an important role in coronavirus replication, but also plays a crucial role in evading host immunity and triggering additional inflammation in the host. Drugs targeting PLpro may have dual antiviral and anti-inflammatory effects, potentially overcoming some of the therapeutic limitations of Mpro or RdRP inhibitors and providing a scientific approach for humans to better resist the threat of coronavirus.
