Shandong Xinbo helped Novo Shengtai to obtain clinical approval of Class 1 innovative drug STC008 injection
2024/08/13
The Drug Safety Evaluation Research Center of Shandong Xinbo Drug Research Co., Ltd. undertakes the comprehensive safety evaluation of the 1 -type innovative drug STC008 injection, showing that this product has good safety and helps it obtain clinical trial permits.
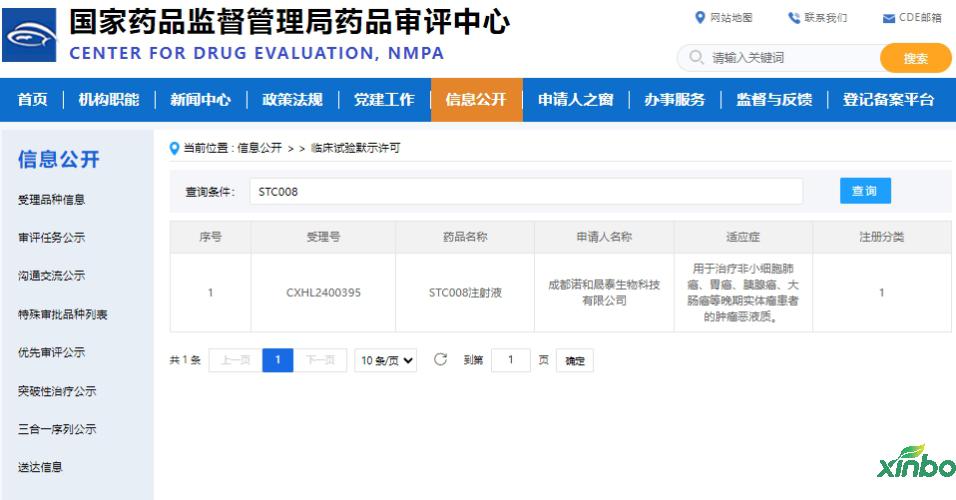
At present, there are no related products in China. The development of this product can meet the clinical needs of patients and bring greater market value. It is expected to become the treatment of tumor vicious liquid therapy of Best-in-Class. Essence
Part of the content is quoted from: https://mp.weixin.qq.com/s/t6g8hqjtluwexzh62cguoq
About Xinbo

Shandong Xinbo Pharmaceutical Research Co., Ltd. is a scientific service enterprise that integrates effective research, toxicology research, drug analysis testing services, animal pathology tests, medical device evaluation services, and is a high -tech enterprise and Shandong enterprise technology Central, Shandong Province Gazelle Enterprise.
The company is located in the economic zone of Beijing, Tianjin, Hebei, and the Economic Development Zone, Texas, which is known as the "Jiuda Sky" and "Shenjing Portal". At present, more than 16,000 square meters of pharmacological and toxicology research laboratories that meet international standards are equipped with more than 800 advanced instruments and equipment at home and abroad. Among them, the toxicology research laboratory can be administered in accordance with the GLP standards of NMPA and FDA. Toxic tests, repeated medication tests, safety pharmacological tests, reproductive toxicity tests, genetic toxic tests, local toxic tests, immunogenic tests, toxic dynamics tests, and carcinogenic tests.
In addition, the company has continuously innovated and broke through the barriers, and improved and explored the areas of difficulty in young animals, continuous administration technology, and tracheal administration technology, and matured into safety evaluations in the fields of pads. The company also pays attention to the needs of the global pharmaceutical industry's innovation and development. Based on the key links of innovative drug research and development, with the experience accumulated by serving the domestic and foreign pharmaceutical industry, it provides a full range of new drug development services for the global pharmaceutical industry.

