Immunogenicity Study
2024/10/24
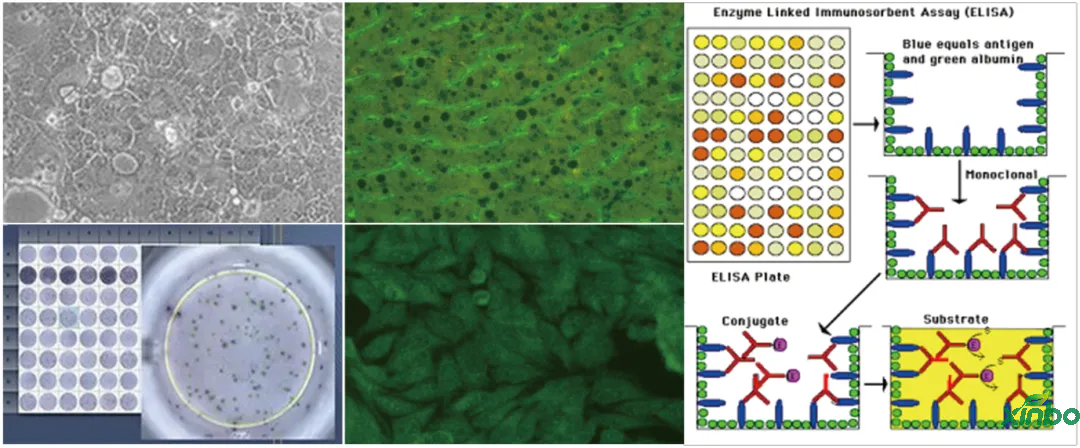
■ Immunogenic (ADA) methodology development: indirect ELISA, bridging ELISA, ACEELISA, etc
■ Anti nuclear antibody testing
■ Enzyme linked immunosorbent spot assay (ELISPOT)
■ Soft agar cloning test
Advantages
>Based on the characteristics of the drug, design personalized testing plans, establish and validate methodologies.
>Adopting a multi-level detection strategy for sample detection, screening tests and confirmation tests are conducted sequentially. If necessary, titer tests and/or neutralization activity tests are performed on confirmed positive samples.
>The laboratory has successfully tested the immunogenicity of dozens of drugs, and based on this, established detection methods for various macromolecular drugs such as peptides, short peptides, monoclonal antibodies, and conjugated antibodies. Monoclonal antibody drugs can have drug resistance of up to 600 µ g/ml or higher under specific experimental conditions.
>Having a comprehensive in vitro cytokine release detection platform. Using human whole blood or peripheral blood mononuclear cells to evaluate cell activation and cytokine release in vitro can to some extent compensate for the deficiency of animal models that cannot fully simulate the human immune stimulation process due to species differences.
