Research on Safety Evaluation of Medical Devices
2024/10/25
In vitro cytotoxicity test
■ Whole body toxicity test
■ Local reaction test after implantation
■ Stimulus and delayed type hypersensitivity test
■ Genetic toxicity test
■ Reproductive toxicity test
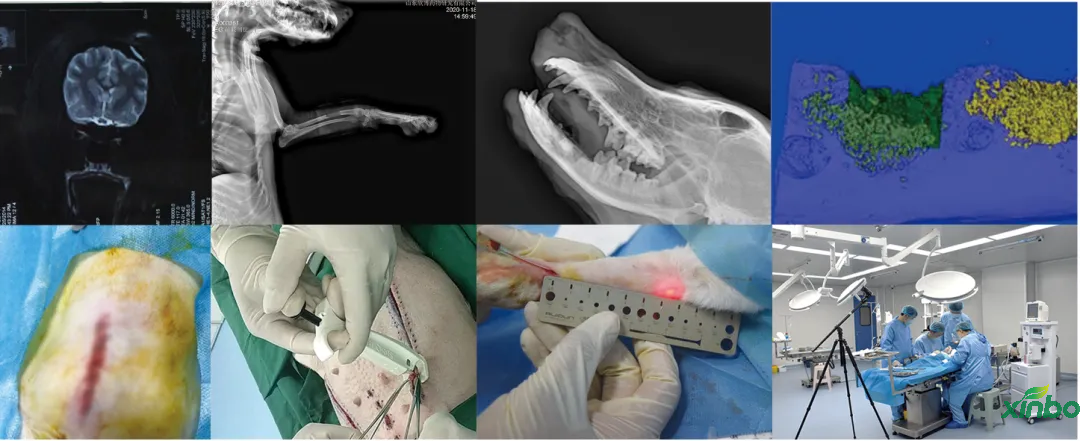
■ Preclinical animal safety and efficacy testing of medical devices
■ Non clinical animal trials of drug device combination projects
Animal species: rat, rabbit, dog, pig, monkey, sheep
Advantages
We have completed non clinical animal trials of high-end medical devices in GLP laboratories in accordance with regulatory requirements, and have conducted animal studies on the safety and effectiveness of multiple Class III medical devices, including in vivo hemostatic materials, neurosurgical hemostatic materials, intravascular occlusion or hemostatic materials, dental implant materials, orthopedic implant materials, new skin suture materials, etc., and have passed the review.