Shandong Xinbo helps Aafa Biology obtaining respiratory tract sympathetic virus MRNA vaccine clinical approval
2024/08/09
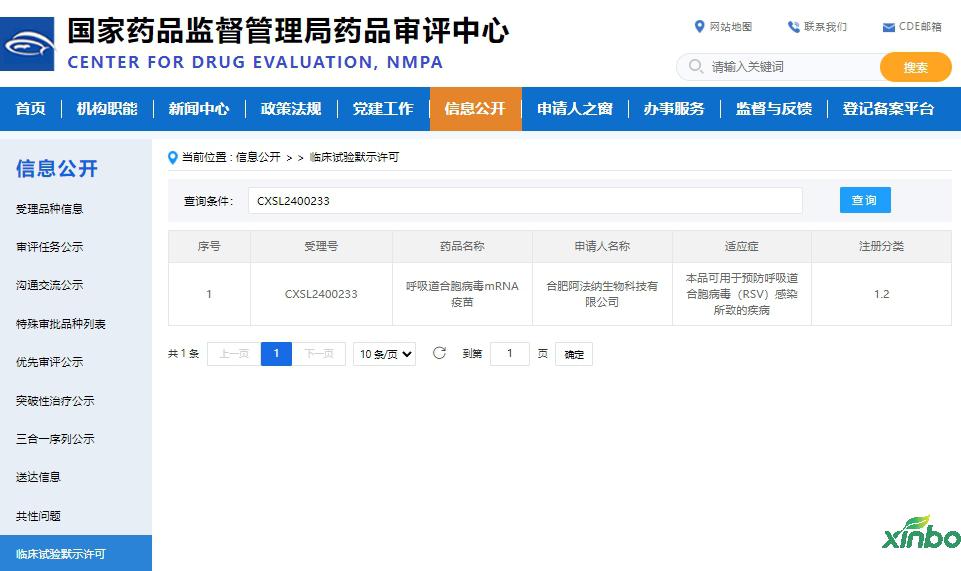
On July 5, 2024, Hefei Afana Biotechnology Co., Ltd. independently developed a Class 1.2 biological product - respiratory syncytial virus mRNA vaccine (AFN0205), which was approved for clinical trials by the National Medical Products Administration (acceptance number: CXSL2400233).
As an important partner of Afana Biosciences in the research and development process, the Drug Safety Evaluation Research Center of Shandong Xinbo Pharmaceutical Research Co., Ltd. strictly follows the guidelines of NMPA and ICH. With its rich project experience and mature technology platform, it undertakes the full set of preclinical safety evaluation research and pharmacokinetic research for RSV mRNA vaccine, helping Afana Biosciences obtain the clinical trial license for RSV mRNA vaccine smoothly.
As an important partner of Afana Biosciences in the research and development process, the Drug Safety Evaluation Research Center of Shandong Xinbo Pharmaceutical Research Co., Ltd. strictly follows the guidelines of NMPA and ICH. With its rich project experience and mature technology platform, it undertakes the full set of preclinical safety evaluation research and pharmacokinetic research for RSV mRNA vaccine, helping Afana Biosciences obtain the clinical trial license for RSV mRNA vaccine smoothly.

- Pre:Pharmacology and toxicology can grasp the inheritance innovation and walk away | Professor Liu Zhaoping, Shandong Xinto, was invited to participate in the first Chinese medicine researcher conference
- Next:Good News | Shandong Xinbo Safety Assessment Center GLP Certification Approval Document Old Certificate Renewed Certificate
