Good news | Warm congratulations to Shandong Xinbo Pharmaceutical Technology Co., Ltd. for obtaining the GLP certification qualification from the National Medical Products Administration!
2025/07/24
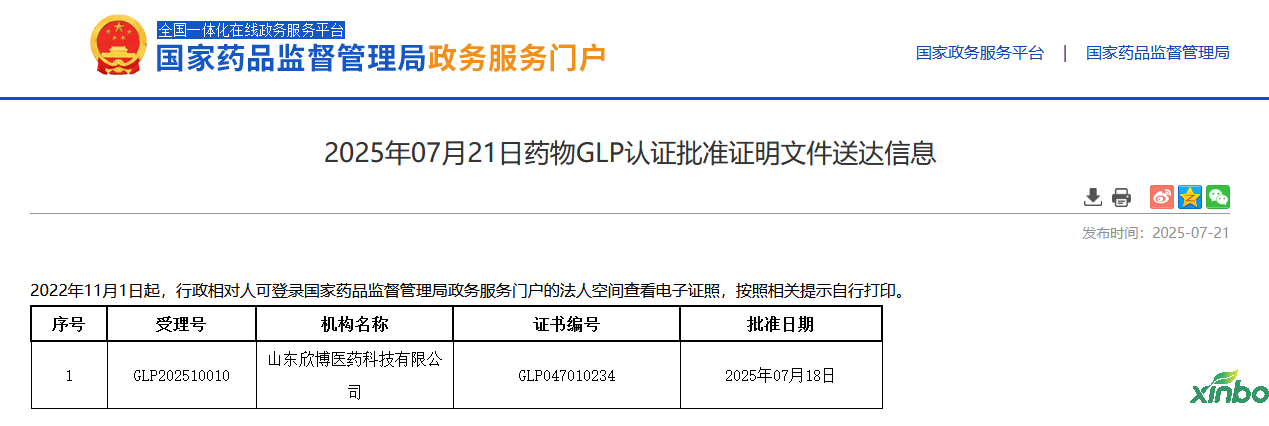
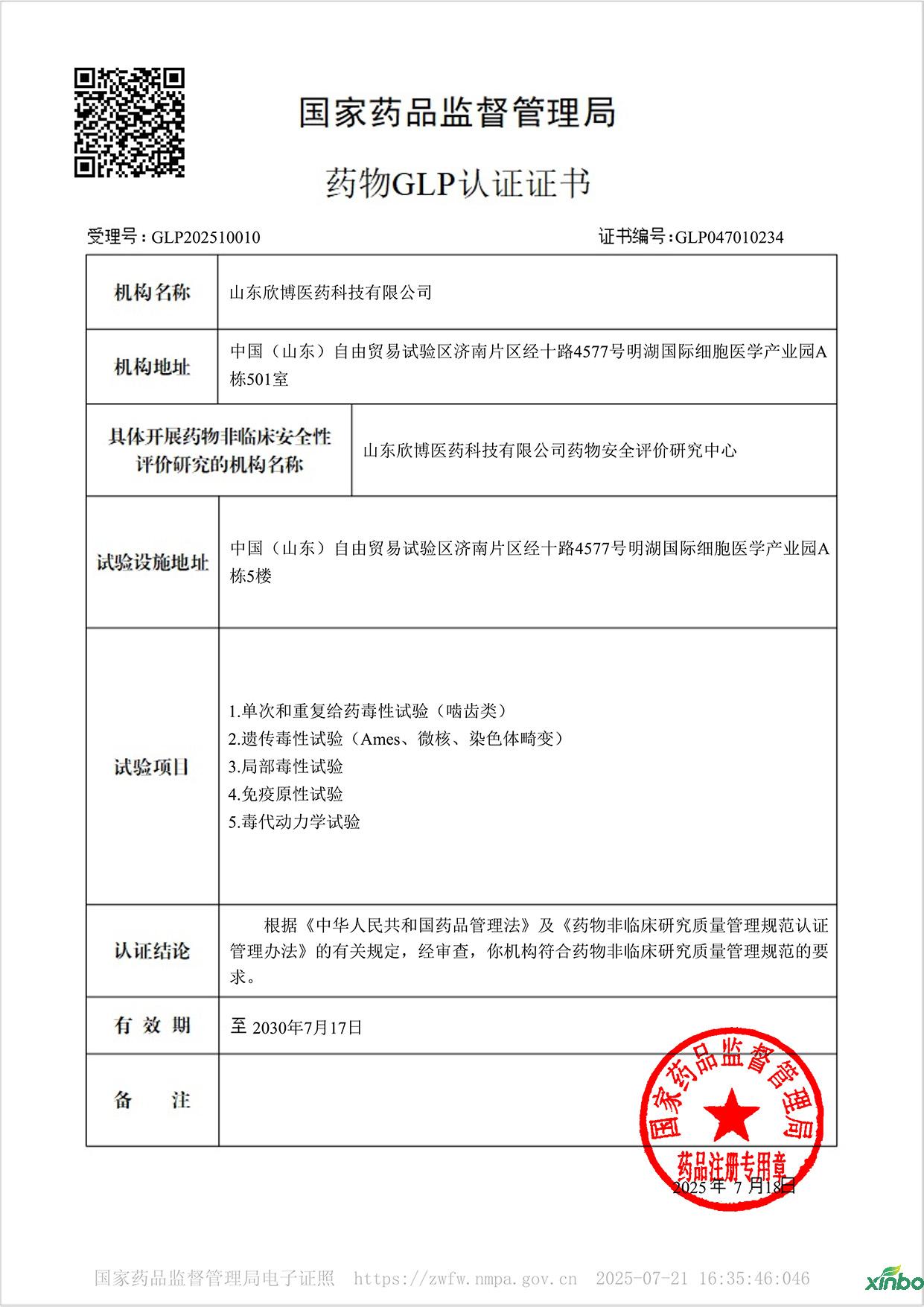
On July 18, 2025, Shandong Xinbo Pharmaceutical Technology Co., Ltd. successfully passed the GLP certification of the National Medical Products Administration and obtained the new version of the GLP certification certificate (certificate number GLP047010234) issued by the National Medical Products Administration. It has obtained five qualifications for single and repeated dose toxicity tests (rodents), genetic toxicity tests (Ames, micronucleus, chromosomal aberration), local toxicity tests, immunogenicity tests, and toxicokinetics tests!




Blood analyzer 6500+ mass spectrometer wax staining machine high-performance liquid chromatograph

In May 2025, NMPA organized an expert review team to conduct a comprehensive and systematic on-site inspection of Xinbo Pharmaceutical. The review experts conducted in-depth evaluations on core elements such as organizational structure and personnel management, quality management system, experimental facilities and equipment, and project operation management system. After strict review, the expert group unanimously believes that the "Drug Safety Evaluation Research Center of Shandong Xinbo Pharmaceutical Technology Co., Ltd." meets the requirements of GLP standards in terms of management system, experimental conditions, and professional technology, and has successfully passed the certification.
Xinbo Pharmaceutical will serve as an important port for the development of Shandong Xinbo, and work together with the Linyi base to quickly catch up in multiple tracks, jointly improve technical service capabilities, accelerate the transformation and application of new drugs from laboratory to clinical, and better provide customers with high-level drug safety evaluation services.
