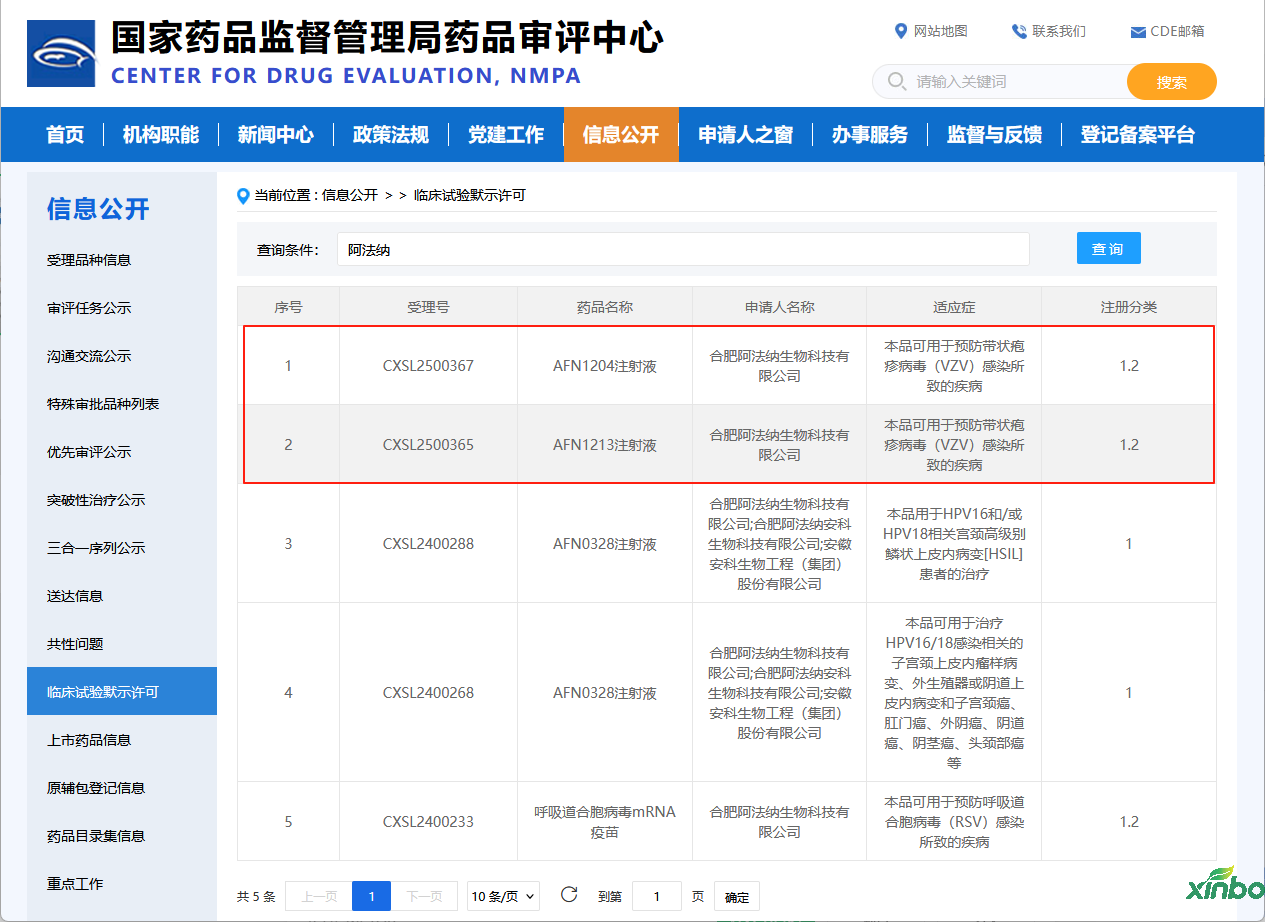
Shandong Xinbo assists Afana Biotech in obtaining clinical approval for two herpes zoster mRNA vaccines
2025/08/06
As an important partner in the research and development process, the Drug Safety Evaluation Research Center of Shandong Xinbo Pharmaceutical Research Co., Ltd. strictly follows the guidelines of NMPA and ICH. With its rich project experience and mature technical platform, it has undertaken preclinical safety evaluation research for the above-mentioned drugs, helping partners obtain clinical trial permits smoothly.
Varicella-Zoster Virus
Herpes zoster is a viral skin disease caused by the reactivation and replication of Varicella Zoster Virus (VZV). It is characterized by clusters of blisters arranged in a unilateral band pattern accompanied by neuralgia. The susceptible population is weak constitution, weakened constitution due to illness and fatigue, weakened immune system, and elderly population.
The VZV virus enters the human body through the respiratory mucosa, spreads through the bloodstream, and causes chickenpox on the skin. It may also have a serious impact on the overall health of patients, causing extremely severe pain and seriously affecting their quality of life.
AFN1204 and AFN1212, as mRNA vaccines, can efficiently activate humoral and cellular immunity without the risk of live viruses. They have advantages in safety, provide stronger immune barriers, and are easy to administer. They can effectively compensate for the shortcomings of traditional inactivated vaccines in immunization applications.
Shandong Xinbo's recent experience in nucleic acid vaccine research