Strength certification | Shandong Xinbo rat sperm morphology examination ability verification obtained "satisfactory" results
2025/12/03
The "satisfactory" result obtained in the sperm testing capability verification of China National Institute for Food and Drug Control is not only an authoritative confirmation of Shandong Xinbo's technical strength, but also sets a professional service benchmark for the field of reproductive toxicity testing.

Shandong Xinbo Reproductive Toxicity Testing Service Project:
Shandong Xinbo has obtained authoritative certification from the China Academy of Food and Drug Control for its three core reproductive toxicity tests: fertility and early embryo development toxicity test (stage I, rats), embryo and fetal development toxicity test (stage II, rats, rabbits), and perinatal development toxicity test (stage III, rats). With its advantages of precise technology, dual-mode parallel, and full cycle tracking, it provides customers with scientific and compliant testing plans that can effectively reduce research and development risks, save costs, and provide full cycle technical support, assisting in product safety research and development and market launch.
Shandong Xinbo Reproductive Toxicity Testing Characteristic Technology:
✅ Milk collection technology: Establish a technology that can repeatedly collect maternal milk to meet pharmacokinetic/toxicokinetic analysis requirements.
✅ B-ultrasound examination of pregnant monkeys
✅ Monkey cub X-ray bone examination
✅ Techniques for examining behavioral indicators of neonatal mice
✅ Fetal abdominal vein blood collection technique
✅ Home free middle ear artery collection technology
✅ Vaginal smear and sperm examination techniques
✅ Bone and visceral examination techniques for fetal mice and rabbits
According to the research and development progress of the variety, the project leader will carry out scientific and reasonable design, and can complete the reproductive stage I and II experiments within 3 months, and the reproductive stage III experiment within 5 months, and provide materials that meet the application requirements.
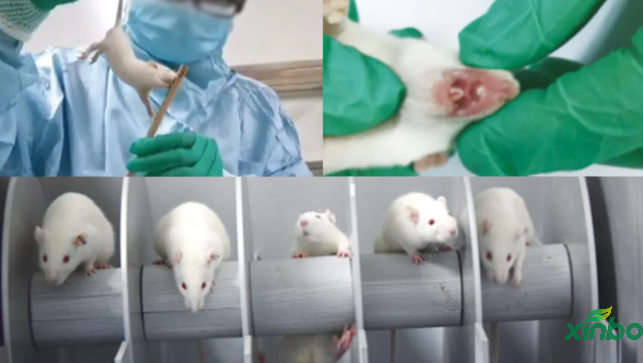
In the future, Shandong Xinbo will continue to deepen its research in reproductive toxicity, continuously upgrade its technology and services, and provide more accurate and comprehensive technical capabilities to safeguard the development of new drugs.

- Pre:Strength Certification | Shandong Xinbo's Bone Marrow Micronucleus Morphological Examination Ability Verification Achieves' Satisfactory 'Results
- Next:Strength Certification | Shandong Xinbo has received 7 "satisfactory" evaluations for ability verification, demonstrating its professional hard power!
