Reproductive Toxicology Study
 Service items
Service items
- ■ Fertility and early embryonic development toxicity study (Segment I, rats)
- ■ Embryo-fetal development toxicity study (Segment II, rats and rabbits)
- ■ Prenatal and postnatal development toxicity study (Segment III, rats)
 Featured Technology
Featured Technology
 Equipment
Equipment
>Established techniques for repeated collection of maternal milk, suitable for pharmacokinetic/toxicokinetic analysis.
>Ultrasound examination of pregnant monkeys.
>X-ray skeletal examination of monkey infants.
>Behavioral indicator assessment techniques for young rodents.
>Fetal abdominal vein blood sampling techniques.
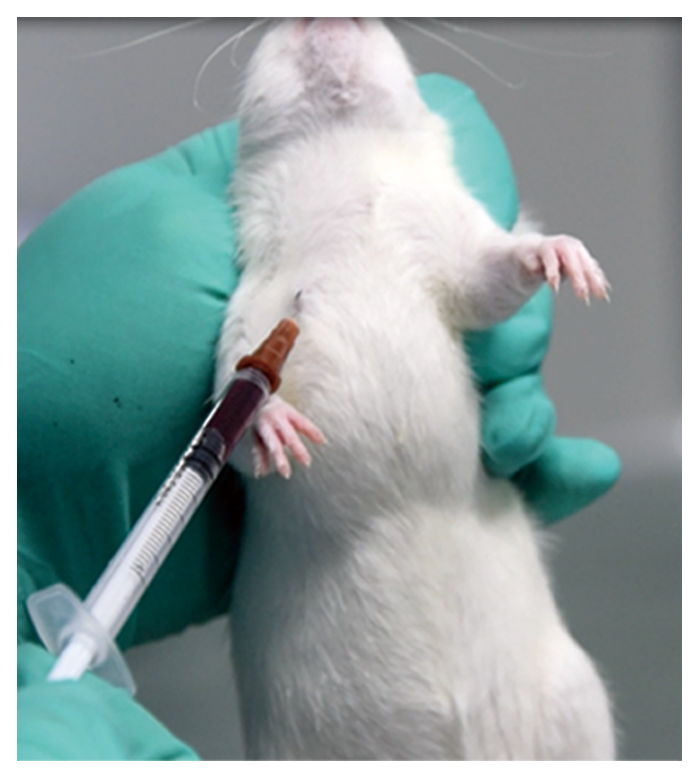
>Auricular artery sampling techniques in domestic rabbits.
>Vaginal smear and sperm examination techniques.
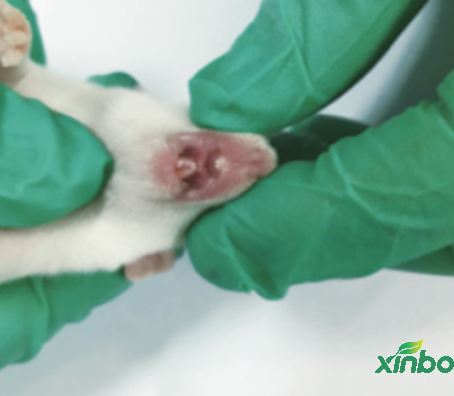
>Skeletal and visceral examination techniques for fetal rodents and rabbits.
>Ultrasound examination of pregnant monkeys.
>X-ray skeletal examination of monkey infants.
>Behavioral indicator assessment techniques for young rodents.
>Fetal abdominal vein blood sampling techniques.
>Auricular artery sampling techniques in domestic rabbits.
>Vaginal smear and sperm examination techniques.
>Skeletal and visceral examination techniques for fetal rodents and rabbits.
 Service advantages
Service advantages
Based on the development progress of the product, the study director carries out scientific and reasonable design. The reproductive toxicity studies for Segment I and II can be completed within 3 months, while the Segment III study can be completed within 5 months, and the required documentation for regulatory submission will be provided accordingly.